Delivery Model
The Mission Statement for the NGDI-UBC is “To develop interventions for neglected global diseases and ensure their delivery to those in need.” The Working Group recognizes that creating new interventions for use on NGD’s is important, and to create real impact they must also plan their work with a model that addresses the complexities and ensures access to those interventions. Towards this end, the NGDI-UBC has promoted the use of a Model of Collaboration that integrates discovery, development and delivery to achieve better health outcomes.
Model of Collaboration
The NGDI-UBC model of Collaboration is partially adapted from Frost & Reich’s Four A;s framework and the traditional pharmaceutical pipeline.
“Our framework is based on four A’s: architecture, the organizational structure and relationships for access; availability, which emphasizes the supply components of access; affordability, the cost issues for various players; and adoption, which includes demand factors and acceptance.”1
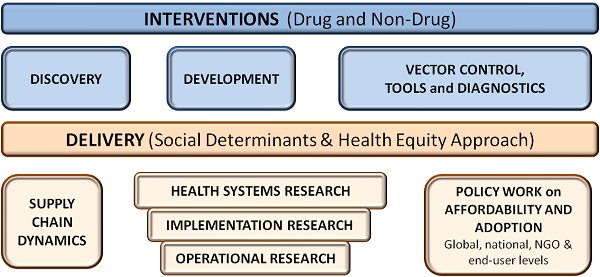
Discovery
- New chemicals
- Reformulations
- Mechanisms of Action
Development
- Pre-clinical
- Target Validation
- Animal Model Testing
- Clinical
- Clinical Trials 1, 2 & 3
- Regulatory Approval
Non-Drug Development
- Vector Control
- Tools
- Diagnostics
Delivery – Supply Chain
- Manufacturing
- Demand Forecasting
- Procurement
- Distribution
- Delivery
Delivery – Adoption
- Health Systems Research – Health financing, governance and policy. Problems pertaining to structuring, management, planning, human resources, service delivery, and quality of care.
- Implementation Research – Multidisciplinary research for new or available health interventions to improve access and the use by population in need.
- Operational Research – To develop solutions to current operational problems of specific health program or specific service delivery component of the health system.
Policy Work on Affordability and Adoption
1. LJ Frost, MR Reich. (2008). ACCESS: How do good health technologies get to poor people in poor countries? Chapter 2: The Access Framework. Harvard Series on Population and International Health. Pp. 16. 2. JHF Remme, T Adam, F Becerra-Posada, C D’Arcangues, M Devlin, et al. (2010) Defining research to improve health systems. PLoS Med 7(11); e1001000. Doi:10.1371/journal.pmed.1001000.